Cincinnati Children’s Hospital
- US regulators have OK'd Pfizer-BioNTech's coronavirus vaccine for use in teenagers.
- The two-dose shot is the first vaccine to be authorized for use in the 12- to 15-year-old age group.
- US health officials have said they will be ready to soon start giving the shot to kids.
- See more stories on Insider's business page.
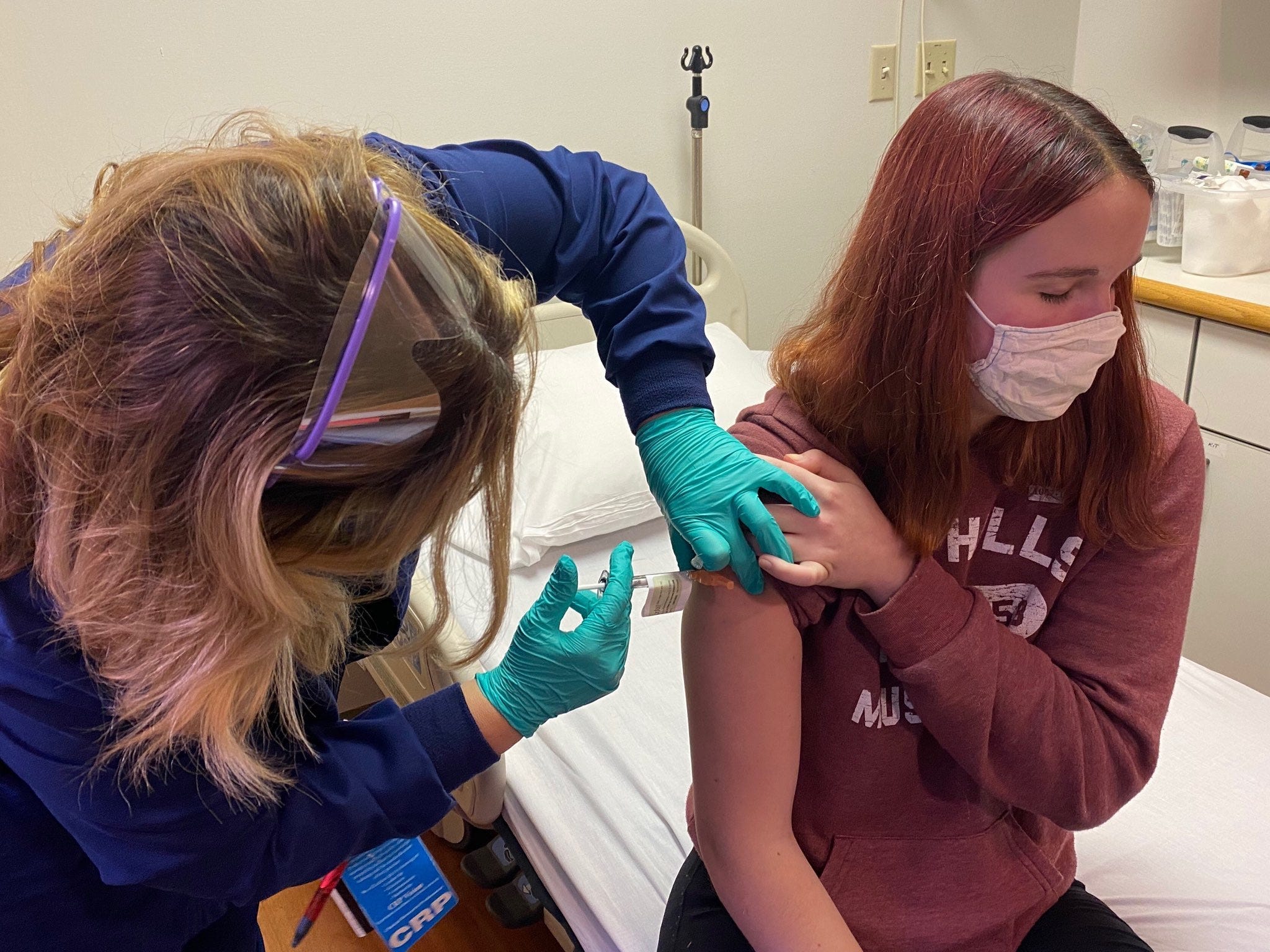
Teenagers in the US are now eligible for a leading coronavirus vaccine, as the Food and Drug Administration on Monday expanded the OK for Pfizer-BioNTech's COVID-19 shot to include 12- to 15-year-olds.
The two-dose vaccine was OK'd last December for anyone 16 years old and up. US health officials said they are prepared to soon start giving the shot to teenagers.
Canada is the only other country to have OK'd a COVID-19 vaccine for teenagers, authorizing Pfizer's vaccine down to age 12 on May 5.
Expanding eligibility to include teenagers could give the immunization campaign a boost. The US has seen the daily rate of people getting their first dose plummet since early April, from a peak of 1.9 million people per day to about 830,000 per day, according to CDC data.
"Today's action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic," said Dr. Janet Woodcock, acting commissioner of the FDA, in a statement. "Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations."
A major driver for that decline has been the fact that a majority of adults in the US have already received at least one dose. About 57% of the adult population has received their first dose.
Pfizer tested the vaccine in a clinical study enrolling 2,260 children aged 12-15. Half of the volunteers received Pfizer's shot, while the other half got a placebo injection. Overall, the trial recorded 18 COVID-19 cases, all in the placebo group. Side effects of the shot were in line with those observed in people 16-25 years old, Pfizer said.
Pfizer, and other vaccine developers, also testing COVID-19 shots in younger populations down to 6-month-olds
The New York drugmaking giant is still testing the shot in younger ages, hoping to further expand its eligibility later this year.
In September, Pfizer anticipates study results showing whether or not the shot works in children aged 2-11.
The final step down for kids - from 6-month-old babies to 2-year-olds - should produce data in November, according to Pfizer's projections.
Other leading vaccine developers are also testing their shots in younger populations.
On May 6, Moderna said its vaccine was highly effective in an initial review of a clinical trial enrolling more than 3,000 teenagers. The Massachusetts biotech said it is "in discussions with regulators about a potential amendment to its regulatory filings" following those early results. Moderna is also enrolling volunteers for a study testing the vaccine in kids 11 years old all the way down to 6 months.
Johnson & Johnson also started testing in April its single-dose vaccine in adolescents 12-17 years of age.
